OEM/ODM Supplier Kit LAL - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM/ODM Supplier Kit LAL - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
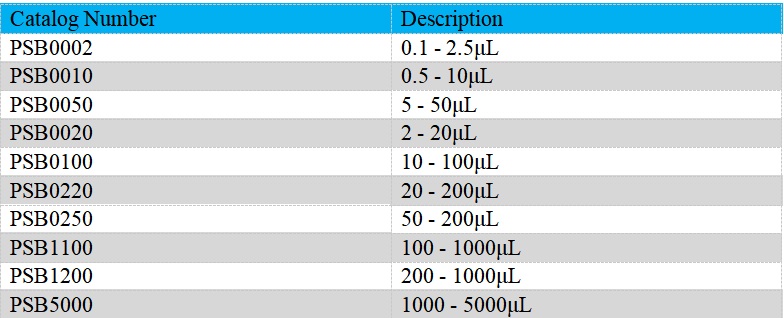
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
To meet the customers' over-expected gratification , we have our robust crew to offer our best over-all support which includes marketing, income, coming up with, production, excellent managing, packing, warehousing and logistics for OEM/ODM Supplier Kit LAL - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Mauritius, Iran, Milan, If you are for any reason unsure which product to select, do not hesitate to contact us and we will be delighted to advise and assist you. This way we will be providing you with all the knowledge needed to make the best choice. Our company strictly follows "Survive by good quality, Develop by keeping good credit. " operation policy. Welcome all the clients old and new to visit our company and talk about the business. We are looking for more and more customers to create the glorious future.
In China, we have purchased many times, this time is the most successful and most satisfactory, a sincere and realiable Chinese manufacturer!