Personlized Products Bacterial Endotoxin Limit For Injection - LAL Reagent Water (Water for Bacterial Endotoxins Test) – Bioendo
Personlized Products Bacterial Endotoxin Limit For Injection - LAL Reagent Water (Water for Bacterial Endotoxins Test) – Bioendo Detail:
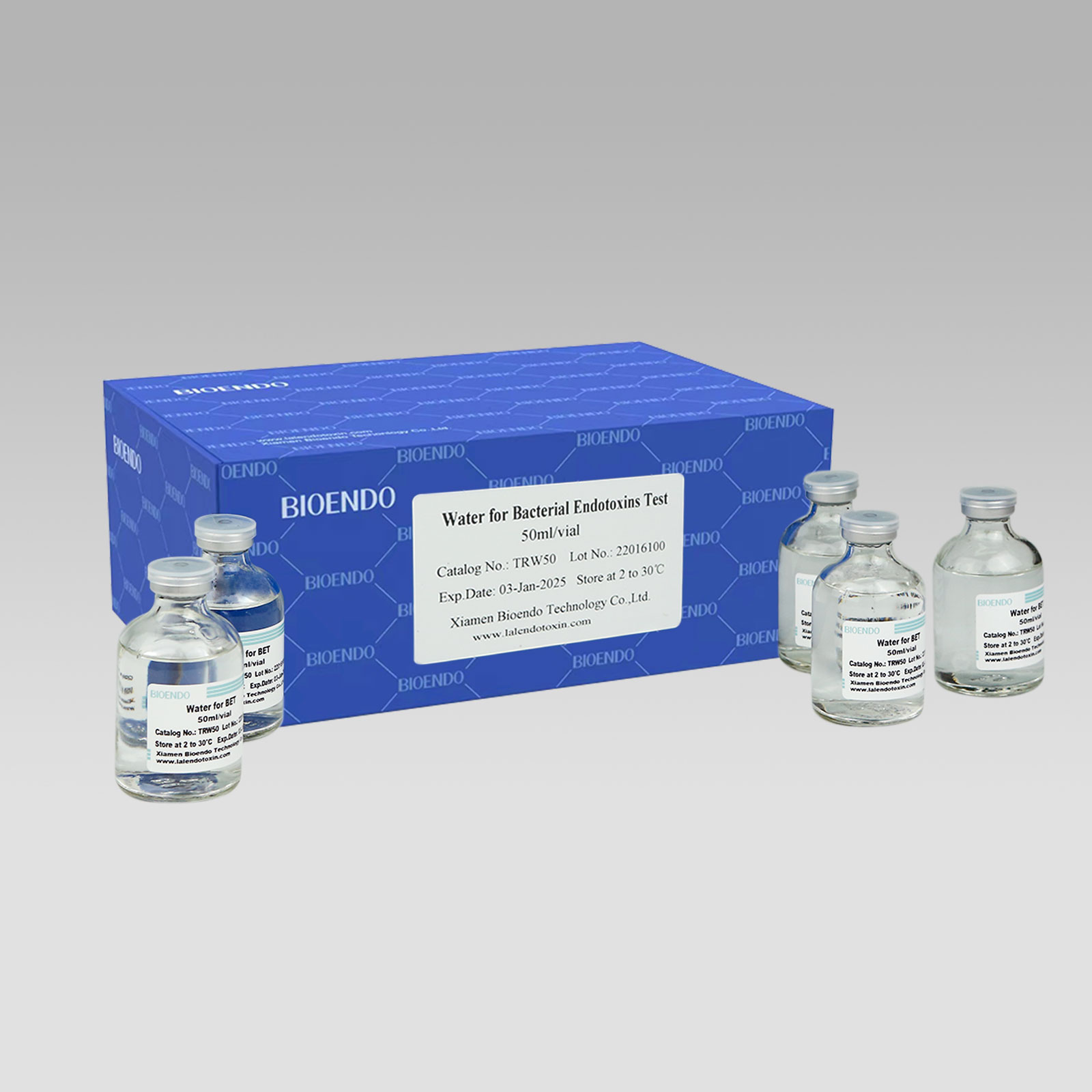
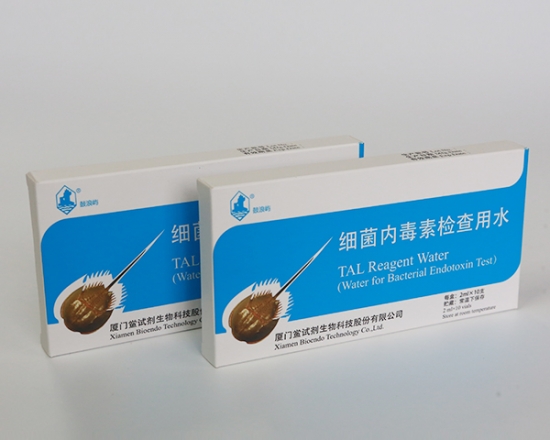
LAL Reagent Water ( Water for Bacterial Endotoxins Test / Water for BET / TAL Reagnt Water )
1. Product Information
LAL Reagent Water (Water for Bacterial Endotoxins Test or BET Water or Water for BET )is specially processed super-purified endotoxin free water is used for Endotoxin test. Its endotoxin concentration is less than 0.005 EU/ml. Various packages, such as 2ml, 10ml, 50ml, 100ml and 500ml per unit, are provided for users’ convenience. LAL Reagent Water (Water for BET) could be used to dilute the assay sample, construct the stand curve, or reconstitute the Lyophilized Amebocyte Lysate reagents.
2. Product Parameter
Endotoxin level: ≤0.005 EU/ml
Water for Bacterial Endotoxins Test is specially processed water is used for endotoxin detection. Its endotoxin concentration is less than 0.005EU/ml. We also provide water for BET with endotoxin level less than 0.001EU/ml for the assay sensitivity 0.001 to 5EU/ml kinetic chromogenic assay.
3. Product Features and Application
Endotoxin Free Water (Water for BET, LAL Reagent water, Endotoxin free water or BET water) is specially processed water intended for reconstitution of Lyophilized Amebocyte Lysate and Control Standard Endotoxin (CSE), and to dilute samples and control standards in the endotoxin assay operation.
For reconstitution of Lyophilized Amebocyte Lysate or Amebocyte Lysate, dilution of the test samples and Control Standard Endotoxin, preparation endotoxin free buffers, and construction of the standard curve. 500ml BET water main apply in extract endotoxins from medical devices.
|
Catalog No. |
Volume (ml/vial) |
Package |
|
TRW02 |
2ml in Ampoule |
In Ampoule, 10 Ampoules/Pack |
|
TRW05 |
5ml in Ampoule |
In Ampoule, 10 Ampoules/Pack |
|
TRW10 |
10ml in Ampoule |
In Ampoule, 10 Ampoules/Pack |
|
TRW50 |
50ml in Glass Vial |
In Glass Vial, 10 Vials/Pack |
|
TRW100 |
100ml in Glass Vial |
In Glass Vial, 10 Vials/Pack |
|
TRW500 |
500ml in Glass Bottle |
1 Vial |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
What is BET water (bacterial endotoxin test water)?
Bacterial endotoxin test water that is endotoxin free level water, it mainly use for endotoxin test assay in the operation of reconstitution and dilution.
Such as, reconstitute lyophilized lysate reagent, control standard endotoxin dilution reconstitution and dilution, and samples dilutions.
Bioendo BET water (Water for BET) can be named TAL reagent water or LAL reagent water. LAL (lyophilized amebocyte lysate).
Product detail pictures:

Related Product Guide:
Our pursuit and company intention is usually to "Always fulfill our purchaser requirements". We go on to acquire and layout excellent high quality products for both our previous and new consumers and realize a win-win prospect for our customers too as us for Personlized Products Bacterial Endotoxin Limit For Injection - LAL Reagent Water (Water for Bacterial Endotoxins Test) – Bioendo , The product will supply to all over the world, such as: Manchester, Lithuania, Accra, We solution have passed through the national skilled certification and been well received in our key industry. Our specialist engineering team will often be ready to serve you for consultation and feedback. We've been able to also provide you with no cost samples to meet your needs. Best efforts are going to be produced to supply you the very best service and solutions. For anyone who is considering our business and solutions, please speak to us by sending us emails or get in touch with us right away. As a way to know our items and enterprise. lot more, you'll be able to come to our factory to find out it. We'll constantly welcome guests from around the globe to our firm. o build enterprise. elations with us. You should really feel absolutely free to make contact with us for small business and we believe we'll share the top trading practical experience with all our merchants.
This company can be well to meet our needs on product quantity and delivery time, so we always choose them when we have procurement requirements.