PriceList for Pyrogen Free Tube - Rapid Gel Clot 10 Samples Kit – Bioendo
PriceList for Pyrogen Free Tube - Rapid Gel Clot 10 Samples Kit – Bioendo Detail:
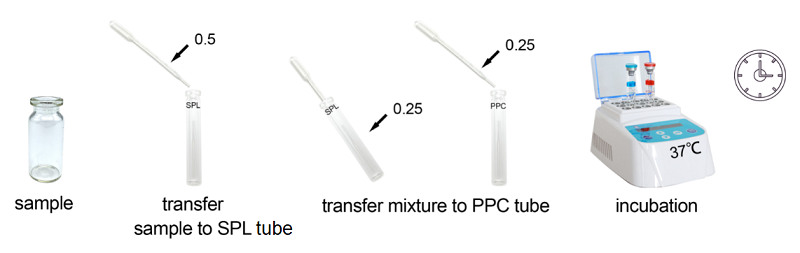
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, RG kit’s result could be gained within 30 minutes. Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need the multi steps’ dilution of Control Standard Endotoxin and test samples. Operation procedures are very convenient, additional experimental equipment are required. It is a convenient and rapid way to detect endotoxin in especial suitable for water or dialysate.
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
10 sample tests in the kit.
Assay time: less than 30 minutes
3. Product Application
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate as well as do quickly endotoxin detection in life science research.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG10025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, 10 Samples Kit |
10 SPL Tubes; 10 PPC Tubes; 10 Endotoxin-free Sample Bottles; 10 Packs of (3pcs Transfer Pipettes) |
0.03 |
≤60 |
|
RG10025006 |
0.06 |
≤60 |
||
|
RG100250125 |
0.125 |
≤45 |
||
|
RG10025025 |
0.25 |
≤30 |
||
|
RG10025050 |
0.5 |
≤30 |
Product detail pictures:



Related Product Guide:
As for aggressive costs, we believe that you will be searching far and wide for anything that can beat us. We can state with absolute certainty that for such high-quality at such rates we have been the lowest around for PriceList for Pyrogen Free Tube - Rapid Gel Clot 10 Samples Kit – Bioendo , The product will supply to all over the world, such as: Ukraine, Chicago, Malta, Our products are mainly exported to Europe, Africa, America, the Middle East and Southeast Asia and other countries and regions. We have enjoyed a great reputation among our customers for quality products and good services.We would make friends with businessmen from at home and abroad, following the purpose of "Quality First, Reputation First, the Best Services."
The factory technical staff not only have high level of technology, their English level is also very good, this is a great help to technology communication.