Quantitative Endotoxin Assay Solution For Bioproducts
Biological Products (Kinetic Chromogenic Method)
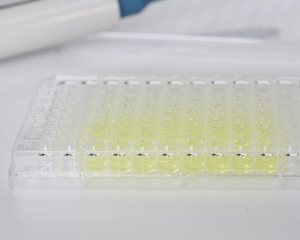
The endotoxin detection kit (kinetic chromogenic method) combines the advantages of the kinetic turbidimetric lysate reagent and the end-point chromogenic lysate reagent. It can accurately quantify bacterial endotoxin according to the chromogenic reaction and KC kit has strong anti-interference ability. The detection range is up to 5 order of magnitudes, and the sensitivity is up to 0.001EU/ml. It is especially suitable for the detection of biological products, such as endotoxin analysis of vaccines, antibodies, proteins, nucleic acids and other samples. It is equipped with Bioendo's endotoxin test microorganism rapid detection system ELx808, which is convenient to operate and allows multiple samples to be detected simultaneously in high-throughput 96 well microplates. The system automatically detects and analyzes in one step.
Related products in the operation of kinetic chromogenic endotoxin test assay:
KC kit: KC0817, KC0817S, KC5017, KC5017S, KC0828, KC0828S, KC5028, KC5028S.
Endotoxin-free sampling bottle, Catalog number PA10, 10ml volume, bigger volume solution will be provided.
Endotoxin-free test tubes, Catalog number T107540 and T1310018
Endotoxin-free microplates (removable/non-removable), Catalog number MP96 or MPC96
Endotoxin-free tips (1000ul and 250ul), Catalog number PT25096 or PT100096
Microplate reader: ELx808
We offer Bioendo KC endotoxin test kit, ELx808, vortex mixer and pyrogen-free microplate strips for quantitative endotoxin assay solution.