Super Lowest Price BET Test In Pharma - Eight-channel Mechanical Pipette – Bioendo
Super Lowest Price BET Test In Pharma - Eight-channel Mechanical Pipette – Bioendo Detail:
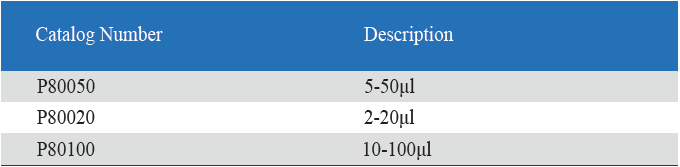
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
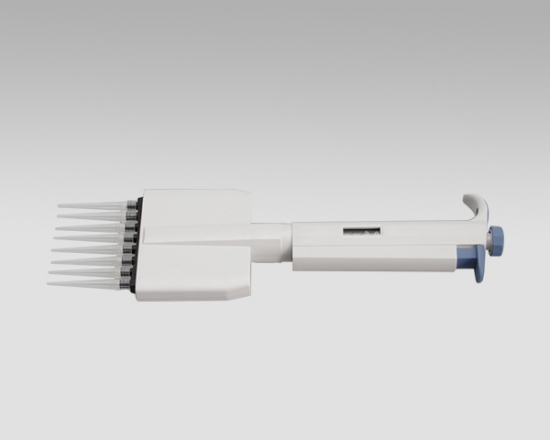
Product detail pictures:
Related Product Guide:
We depend on sturdy technical force and continually create sophisticated technologies to meet the demand of Super Lowest Price BET Test In Pharma - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Houston, Philippines, Afghanistan, Facing fierce global market competition, we have launched the brand building strategy and updated the spirit of "human-oriented and faithful service", with an aim to gain global recognition and sustainable development.
It's really lucky to find such a professional and responsible manufacturer, the product quality is good and delivery is timely, very nice.







