Super Purchasing for Endotoxin Method - LAL Reagent Water (Water for Bacterial Endotoxins Test) – Bioendo
Super Purchasing for Endotoxin Method - LAL Reagent Water (Water for Bacterial Endotoxins Test) – Bioendo Detail:
LAL Reagent Water ( Water for Bacterial Endotoxins Test / Water for BET / TAL Reagnt Water )
1. Product Information
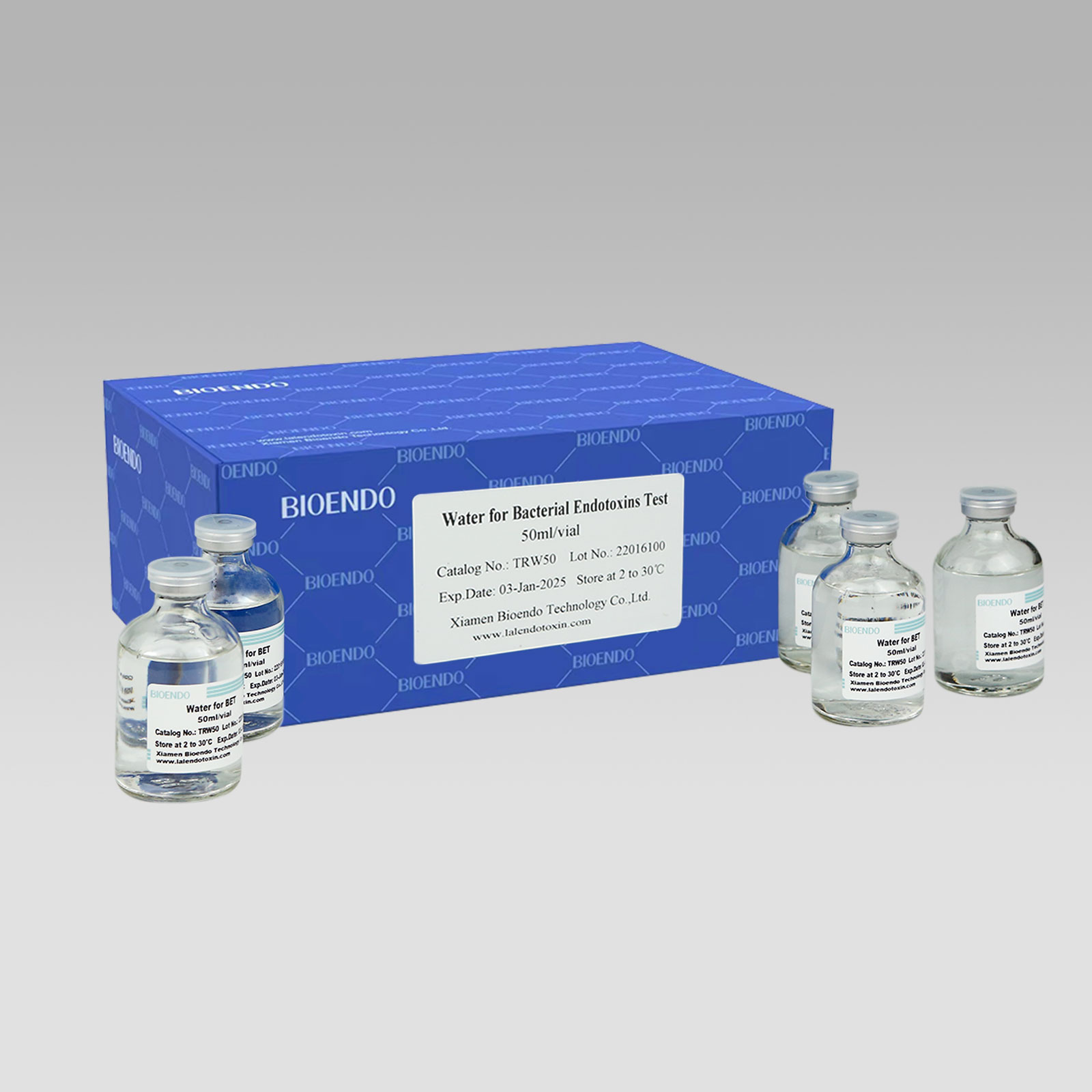
LAL Reagent Water (Water for Bacterial Endotoxins Test or BET Water or Water for BET )is specially processed super-purified endotoxin free water is used for Endotoxin test. Its endotoxin concentration is less than 0.005 EU/ml. Various packages, such as 2ml, 10ml, 50ml, 100ml and 500ml per unit, are provided for users’ convenience. LAL Reagent Water (Water for BET) could be used to dilute the assay sample, construct the stand curve, or reconstitute the Lyophilized Amebocyte Lysate reagents.
2. Product Parameter
Endotoxin level: ≤0.005 EU/ml
Water for Bacterial Endotoxins Test is specially processed water is used for endotoxin detection. Its endotoxin concentration is less than 0.005EU/ml. We also provide water for BET with endotoxin level less than 0.001EU/ml for the assay sensitivity 0.001 to 5EU/ml kinetic chromogenic assay.
3. Product Features and Application
Endotoxin Free Water (Water for BET, LAL Reagent water, Endotoxin free water or BET water) is specially processed water intended for reconstitution of Lyophilized Amebocyte Lysate and Control Standard Endotoxin (CSE), and to dilute samples and control standards in the endotoxin assay operation.
For reconstitution of Lyophilized Amebocyte Lysate or Amebocyte Lysate, dilution of the test samples and Control Standard Endotoxin, preparation endotoxin free buffers, and construction of the standard curve. 500ml BET water main apply in extract endotoxins from medical devices.
|
Catalog No. |
Volume (ml/vial) |
Package |
|
TRW02 |
2ml in Ampoule |
In Ampoule, 10 Ampoules/Pack |
|
TRW05 |
5ml in Ampoule |
In Ampoule, 10 Ampoules/Pack |
|
TRW10 |
10ml in Ampoule |
In Ampoule, 10 Ampoules/Pack |
|
TRW50 |
50ml in Glass Vial |
In Glass Vial, 10 Vials/Pack |
|
TRW100 |
100ml in Glass Vial |
In Glass Vial, 10 Vials/Pack |
|
TRW500 |
500ml in Glass Bottle |
1 Vial |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
What is BET water (bacterial endotoxin test water)?
Bacterial endotoxin test water that is endotoxin free level water, it mainly use for endotoxin test assay in the operation of reconstitution and dilution.
Such as, reconstitute lyophilized lysate reagent, control standard endotoxin dilution reconstitution and dilution, and samples dilutions.
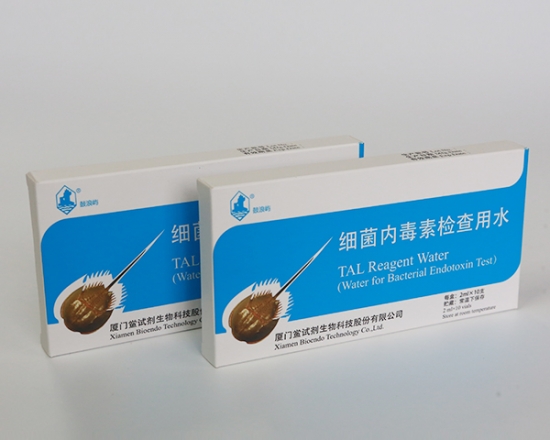
Bioendo BET water (Water for BET) can be named TAL reagent water or LAL reagent water. LAL (lyophilized amebocyte lysate).
Product detail pictures:

Related Product Guide:
We have quite a few great team customers very good at internet marketing, QC, and dealing with kinds of troublesome trouble while in the output approach for Super Purchasing for Endotoxin Method - LAL Reagent Water (Water for Bacterial Endotoxins Test) – Bioendo , The product will supply to all over the world, such as: Macedonia, Grenada, Turkey, We always adhere to follow the honesty, mutual benefit, common development, after years of development and the tireless efforts of all staff, now has perfect export system, diversified logistics solutions, thorough meet customer shipping, air transport, international express and logistics services. Elaborate one-stop sourcing platform for our customers!
In our cooperated wholesalers, this company has the best quality and reasonable price, they are our first choice.