Super Purchasing for Endotoxin Method - Pyrogen-free Pipette tips and Consumables – Bioendo
Super Purchasing for Endotoxin Method - Pyrogen-free Pipette tips and Consumables – Bioendo Detail:
Pyrogen-free Pipette tips and tip box
1. Product information
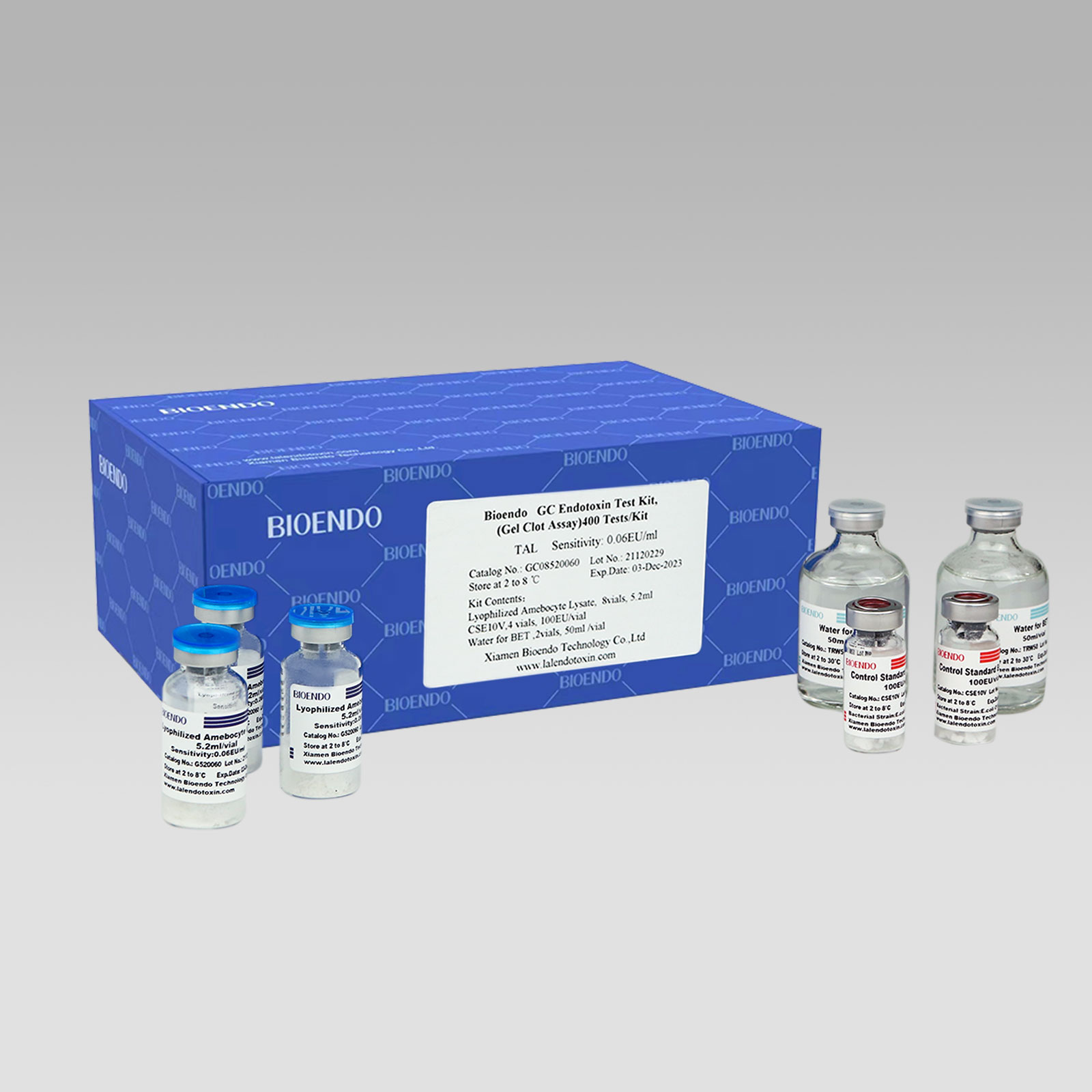
We offer various low endotoxin, pyrogen-free accessories,includes Water for Bacterial Endotoxins Test, endotoxin-free test tubes, pyrogen-free pipette tips, pyroegn-free microplates for your operation. High quality depyrogenated and low endotoxin level consumables to ensure the success of your endotoxin assays.
Pyrogen-free pipette tips are certified to contain <0.001 EU/ml endotoxin. The tips allow more flexibility with different pipettors. The endotoxin-free pipette tips are good at endotoxin testing procedures, such as LAL reagent’s reconstitution, dilution of Control Standard Endotoxin, dilution of test samples, all related operations involves in the bacterial endotoxin test.
2. Product parameter
Top endotoxin free level. Endotoxins less than 0.005 EU/ml.
3. Product features and application
Selection of 4 tips or 5 tips per bag and 96 tips per box. For sample preparation, lysate reagent pipette transfer and dilution of Control Standard Endotoxin.
|
Catalog No. |
Description |
Package |
|
PT2005 |
Pyrogen-free 200μl Pipette Tips |
5 Tips/Pack |
|
PT10004 |
Pyrogen-free 1000μl Pipette Tips |
4 Tips/Pack |
|
PT25096 |
Pyrogen-free 250μl Pipette Tips |
96 Tips/Box |
|
PT100096 |
Pyrogen-free 1000μl Pipette Tips |
96 Tips/Box |
such as endotoxin free tubes; pyrogen-ree tips; pyrogen-free microplates; endotoxin free sample containers;
According to Pharmacopoeia, the endotoxin free consumables are needed in the procedure of endotoxin test assay, such as sample vessel, dilution and reaction tubes, pipette tips, have to choose endotoxin free consumables. the utensils needed for the experiment need to be processed to remove possible exogenous endotoxins. If the endotoxin is not removed, it will interfere with the experiment.
Product detail pictures:


Related Product Guide:
Our personnel are always inside the spirit of "continuous improvement and excellence", and together with the outstanding excellent goods, favorable price and good after-sales services, we try to gain every customer's trust for Super Purchasing for Endotoxin Method - Pyrogen-free Pipette tips and Consumables – Bioendo , The product will supply to all over the world, such as: Belize, Tunisia, California, Our market share of our products has greatly increased yearly. If you are interested in any of our products or would like to discuss a custom order, please feel free to contact us. We are looking forward to forming successful business relationships with new clients around the world in the near future. We are looking forward to your inquiry and order.
The enterprise has a strong capital and competitive power, product is sufficient, reliable, so we have no worries on cooperating with them.