Top Suppliers Dry Heat Sterilizer Validation - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo
Top Suppliers Dry Heat Sterilizer Validation - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo Detail:
Endotoxin and (1,3)-ß-D-glucan assay software
1. Product information
Endotoxin and (1,3)-ß-D-glucan assay software is a powerful kinetic data analysis software,which gives the user data acquisition and processing of the maximum flexibility.
Features:
• Apply to Endotoxin assay,(1,3)-ß-D-glucan assay and ELISA data analysis
• With standard version and clinical diagnostic version for the different user groups.
• Data could be output transitions and connected to the LIS system.
• Customizable endotoxin test reports.
• Data analyzed by onset time, average rate, maximum rate and other methods.
• Data linear fitting or polynomial fitting.
• Real-time backup of the original data read.
• Integration of variety of kinetic microplate readers.
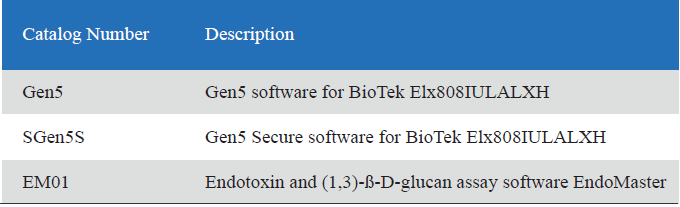
Product detail pictures:


Related Product Guide:
Being supported by an state-of-the-art and skilled IT team, we could supply technical support on pre-sales & after-sales service for Top Suppliers Dry Heat Sterilizer Validation - Endotoxin Assay and (1,3)-ß-D-glucan assay software – Bioendo , The product will supply to all over the world, such as: Iceland, Frankfurt, Israel, Immediate and specialist after-sale service supplied by our consultant group has happy our buyers. Detailed Info and parameters from the merchandise will probably be sent to you for any thorough acknowledge. Free samples may be delivered and company check out to our corporation. n Morocco for negotiation is constantly welcome. Hope to get inquiries type you and construct a long-term co-operation partnership.
The supplier abide the theory of "quality the basic, trust the first and management the advanced" so that they can ensure a reliable product quality and stable customers.