Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettor – Bioendo
Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
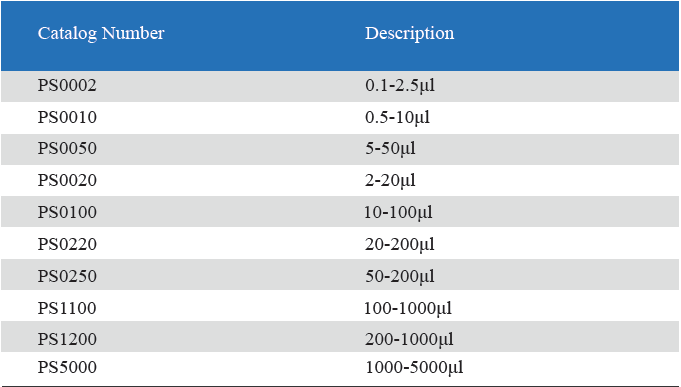
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Well-run equipment, expert income workforce, and far better after-sales expert services; We are also a unified large family, anyone stick to the corporate value "unification, dedication, tolerance" for Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Egypt, India, Holland, With the spirit of "high quality is our company's life; good reputation is our root", we sincerely hope to cooperate with customers from at home and abroad and hope to build a good relationship with you.
Customer service staff and sales man are very patience and they all good at English, product's arrival is also very timely, a good supplier.






