Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettor – Bioendo
Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
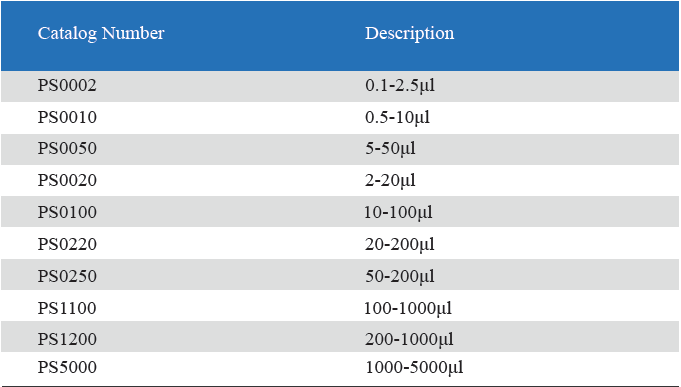
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
To be the stage of realizing dreams of our employees! To build a happier, far more united and far more specialist team! To reach a mutual profit of our customers, suppliers, the society and ourselves for Top Suppliers Dry Heat Sterilizer Validation - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Portugal, Egypt, Estonia, "Create Values,Serving Customer!" is the aim we pursue. We sincerely hope that all customers will establish long term and mutually beneficial cooperation with us.If you wish to get more details about our company, You should contact with us now!
On this website, product categories is clear and rich, I can find the product I want very quickly and easily, this is really very good!








