Wholesale Discount BET Endotoxin Test - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo
Wholesale Discount BET Endotoxin Test - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo Detail:
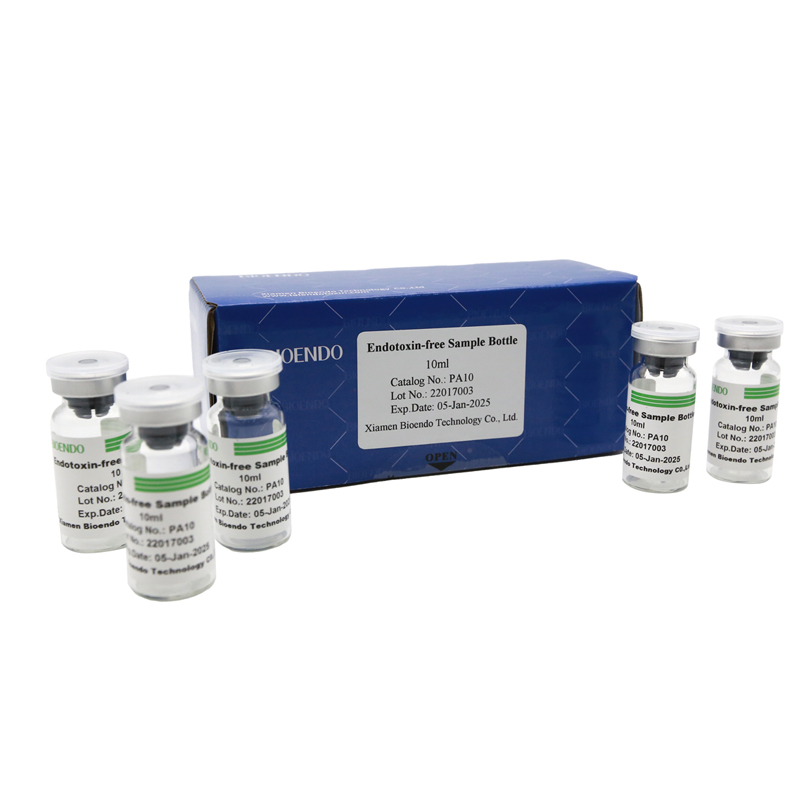
Depyrogenated Sample Container
1. Product information
We offer various of low endotoxin, pyrogen free accessories products,includes Water for Bacterial Endotoxins Test, pyrogen-free test tubes, pyrogen-free pipettor tips, microplates and sample bottles for your conveniences. High quality depyrogenated low endotoxin pyrogen free products insure the success of your experiments.
Depyrogenated (Endotoxin Free) Sample container(endotoxin free bottle, pyrogen free bottle, pyrogen free sample bottle)are glass bottles contain less than 0.005 EU/ml endotoxin. These bottles could be used to store various samples for lps endotoxin test, such as protein solution, vaccines, DNA solutions, dialysate, water for injections, etc., for endotoxin testing. Come with endotoxin free seals.
2. Product parameter
Endotoxin level < 0.005 EU/ml
3. Product features and application
For preparing and storage of the test samples.
| Catalog Number | Descriptions | Package |
| PA2 | Endotoxin free sample glass ampoule, 2ml | 10Pcs/Pack |
| PA10 | Endotoxin free sample glass vial, 10ml | 10Pcs/Pack or 110Pcs/Pack |
| PA50 | Endotoxin free sample glass vial, 50ml | 10Pcs/Pack |
| PA125 | Pyrogen free sample bottle, 125ml | 1Pcs/Pack |
| PA500 | Pyrogen free sample bottle, 500ml | 1Pcs/Pack |
such as endotoxin free tubes; endotoxin free tips; endotoxin free microplates; endotoxin free sample bottles;
According to China Pharmacopoeia, the utensils needed in the procedure of endotoxin test assay, such as sample vessel, dilution and reaction tubes, pipette tips, have to choose endotoxin free consumables. the utensils needed for the experiment need to be processed to remove possible exogenous endotoxins. If the endotoxin is not removed, it will interfere with the experiment.
Product detail pictures:





Related Product Guide:
With this motto in mind, we have turn out to be amongst probably the most technologically innovative, cost-efficient, and price-competitive manufacturers for Wholesale Discount BET Endotoxin Test - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo , The product will supply to all over the world, such as: Turkmenistan, Manchester, Gabon, So that you can utilize the resource from the expanding info in international trade, we welcome shoppers from everywhere on-line and offline. In spite of the good quality solutions we provide, effective and satisfying consultation service is supplied by our professional after-sale service team. Product lists and detailed parameters and any other info weil be sent to you timely for your inquiries. So you should make contact with us by sending us emails or call us if you have any questions about our corporation. ou may also get our address info from our web page and come to our company to get a field survey of our merchandise. We've been confident that we have been going to share mutual achievement and create strong co-operation relations with our companions in this marketplace. We're searching forward for your inquiries.
We are a small company that has just started, but we get the company leader's attention and gave us a lot of help. Hope we can make progress together!







