Wholesale Price China Kinetic Turbidimetric LAL kit for endotoxin detection - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo
Wholesale Price China Kinetic Turbidimetric LAL kit for endotoxin detection - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo Detail:
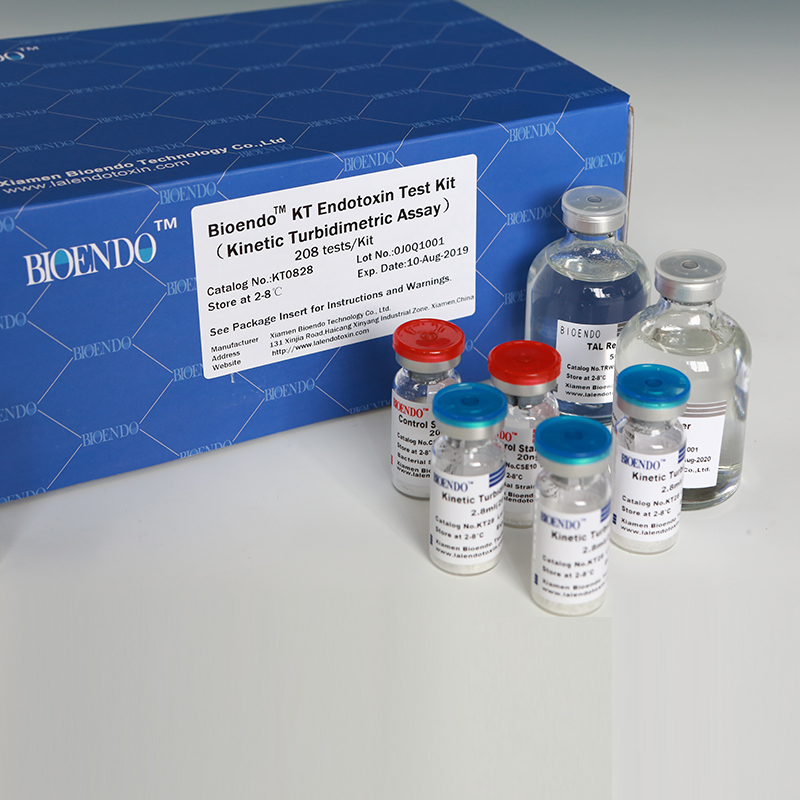
Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay)
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
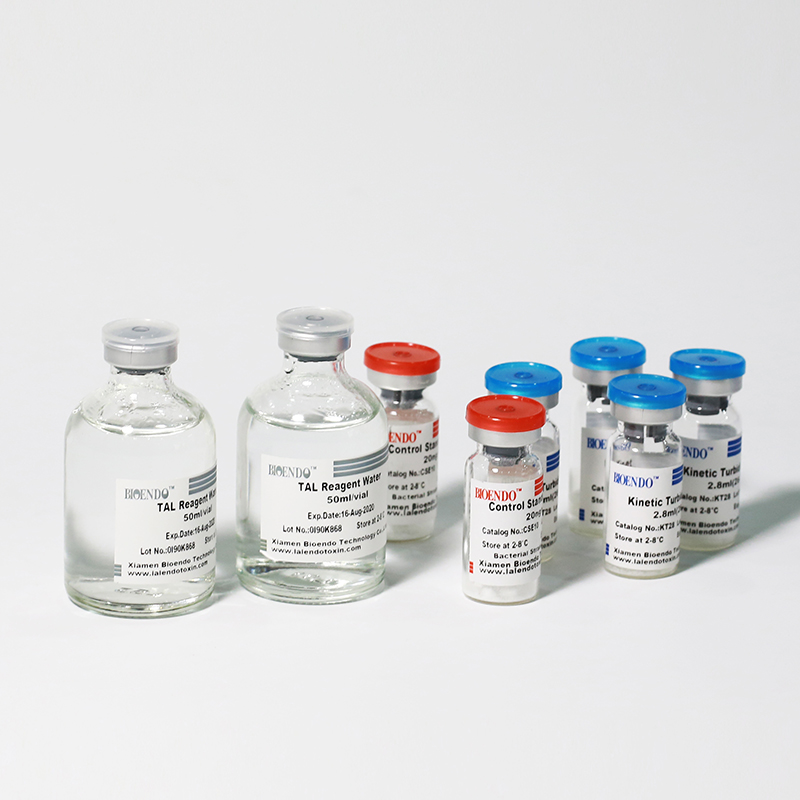
The kit contains Lyophilized Amebocyte Lysate, Control Standard Endotoxin, and Water for BET. KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) require a kinetic microplate reader such as ELx808IULALXH or a kinetic tube reader. Kinetic software is also required for calculation of the endotoxin concentration.
2. Product Parameter
Assay Range: 0.005 – 5EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Waterfor injection endotoxin assay, raw material endotoxin testing or endotoxinlevel monitoring during manufacturing process for pharmaceutical companies ormedical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KT0817 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 128 tests/kit |
8 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0817S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT0852 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 400 Tests/Kit |
8 Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 8 Reconstitution Buffer, 6.0ml/vial; 4 CSE; 3 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0852S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5017 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 800 Tests/Kit |
50 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 50 Reconstitution Buffer, 3.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5017S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5052 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 2500 Tests/Kit |
50 Lyophilized Amebocyte Lysate,5.2ml (50 Tests/vial); 50 Reconstitution Buffer, 6.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5052S |
0.005-5EU/ml, 0.01-10EU/ml |
Product Condition:
The potency of Lyophilized Amebocyte Lysate and Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:



Related Product Guide:
owing to very good support, a variety of high quality merchandise, aggressive costs and efficient delivery, we love an excellent name among the our clients. We are an energetic company with wide market for Wholesale Price China Kinetic Turbidimetric LAL kit for endotoxin detection - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo , The product will supply to all over the world, such as: Mexico, Germany, Albania, With the intensified strength and more reliable credit, we are here to serve our customers by providing the highest quality and service, and we sincerely appreciate your support. We will endeavor to maintain our great reputation as the best products supplier in the world. If you have any questions or comments, please contact with us freely.
Wide range, good quality, reasonable prices and good service, advanced equipment, excellent talents and continuously strengthened technology forces,a nice business partner.







