Wholesale Price China Kinetic Turbidimetric LAL kit for endotoxin detection - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo
Wholesale Price China Kinetic Turbidimetric LAL kit for endotoxin detection - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo Detail:
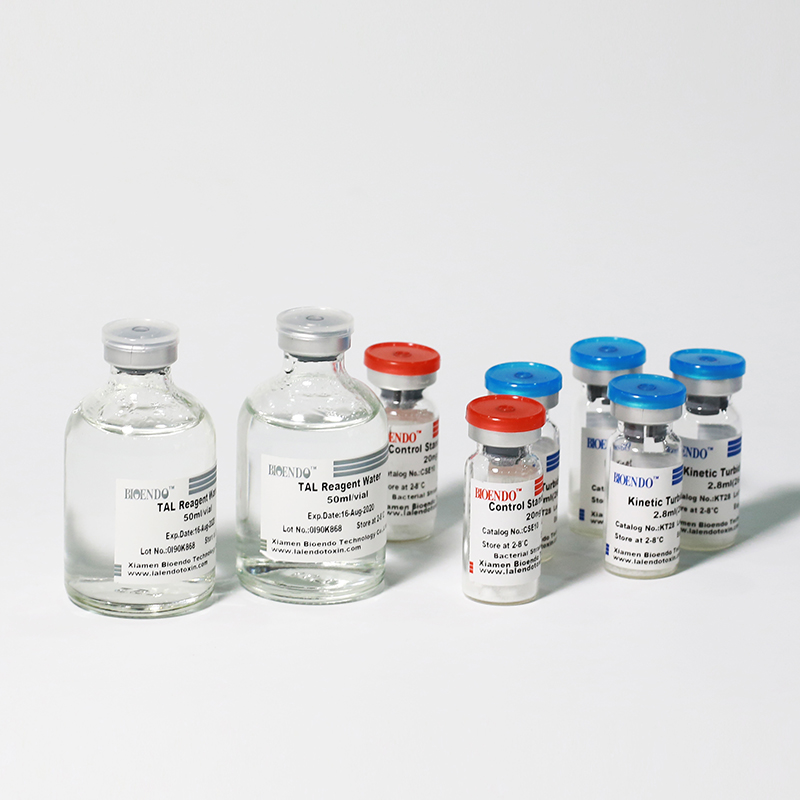
Kinetic Turbidimetric Amebocyte Lysate Vial
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
2. Product Parameter:
Assay range:0.005-50EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
ml/vial |
Tests/Vial |
Vials/Pack |
Sensitivity EU/ml |
|
KT17 |
1.7 |
16 |
10 |
0.01-10EU/ml |
|
KT17S |
1.7 |
16 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
|
KT52 |
5.2 |
50 |
10 |
0.01-10EU/ml |
|
KT52S |
5.2 |
50 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
The Lyophilized Amebocyte Lysate reagent sensitivity and the Control Standard Endotoxin potency are assayedagainst USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come withproduct instruction, Certificate of Analysis.
Product detail pictures:


Related Product Guide:
"Quality initially, Honesty as base, Sincere company and mutual profit" is our idea, in order to create repeatedly and pursue the excellence for Wholesale Price China Kinetic Turbidimetric LAL kit for endotoxin detection - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo , The product will supply to all over the world, such as: Moscow, Nigeria, Eindhoven, We integrate all our advantages to continuously innovate, improve and optimize our industrial structure and product performance. We will always believe in and work on it. Welcome to join us to promote green light, together we will make a better Future!
The after-sale warranty service is timely and thoughtful, encounter problems can be resolved very quickly, we feel reliable and secure.








