Wholesale Price China LAL test for pyrogens - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
Wholesale Price China LAL test for pyrogens - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
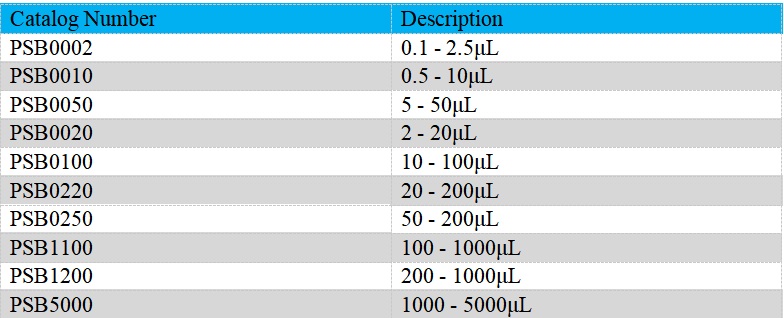
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
"Based on domestic market and expand overseas business" is our improvement strategy for Wholesale Price China LAL test for pyrogens - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Japan, Portugal, Buenos Aires, Our company is an international supplier on this kind of merchandise. We supply an amazing selection of high-quality merchandise. Our goal is to delight you with our distinctive collection of mindful items while providing value and excellent service. Our mission is simple: To supply the best items and service to our customers at the lowest prices possible.
Good quality, reasonable prices, rich variety and perfect after-sales service, it's nice!








